Spravato, a fast-acting treatment for depression based on ketamine, has just become much more affordable in Australia. The medication has been included in the country’s Pharmaceutical Benefits Scheme (PBS), a government program which covers medication costs, as of May 1st, 2025.
With this change, thousands of Australians struggling with treatment-resistant depression (TRD) can now access Spravato at a fraction of the original cost.
Key Takeaways
- Spravato is now available on the PBS, making it much more affordable for Australians with treatment-resistant depression.
- It works quickly by boosting brain connections and restoring balance in areas affected by depression.
- Clinical trials show it’s effective, but it must be used under strict safety rules.
- This move could be the start of a new era in mental health care, with more ketamine-based and psychedelic treatments becoming available in the future.
What Is Spravato?
Spravato is the brand name for a nasal spray medicine that contains esketamine, a derivative of ketamine. Initially developed as an anesthetic, ketamine has proven to be a powerful mental health drug. Esketamine is a more targeted version of ketamine, made from one of its two mirror-image molecules, and Spravato was specifically created to treat depression.
Like ketamine, Spravato interacts with proteins in the brain called NMDA receptors. This interaction increases activity in brain regions associated with mood and emotional regulation. It also increases neuroplasticity, which includes building connections between nerve cells that may be impaired in depression and strengthening neurological circuits.
Unlike regular antidepressants, which can take weeks or months to show effects, Spravato can start working within hours or days. Spravato can alleviate depression symptoms when conventional medication has failed to do so.
Spravato’s Approval and Use in Australia
Spravato was approved by the Therapeutic Goods Administration (TGA), Australia’s medical regulator, for use in people with treatment-resistant depression (TRD) in March 2021.
To qualify for TRD, patients must have tried at least two antidepressants for at least six months and experienced no significant improvement in symptoms. Patients also need to start a new antidepressant at the same time they begin Spravato, and can only take the medicine in approved healthcare settings.
While Spravato has shown promise in clinical trials, its high costs have significantly limited its use in depression care. A single dose can cost between $500 and $900, without even factoring in consulting and monitoring costs.
However, with the TGA now including Spravato under the Pharmaceutical Benefits Scheme (PBS), the price will be sizably reduced for Australians who are eligible for treatment.
Spravato Added to the PBS Increasing Mental Health Access
The PBS is a govenment program which subsidizes the cost of prescription medicines. By being part of this scheme, the price of Spravato has dropped to $31.60 per dose for regular patients and $7.70 per dose for concession card holders.
To get Spravato under the PBS, patients must follow some essential steps:
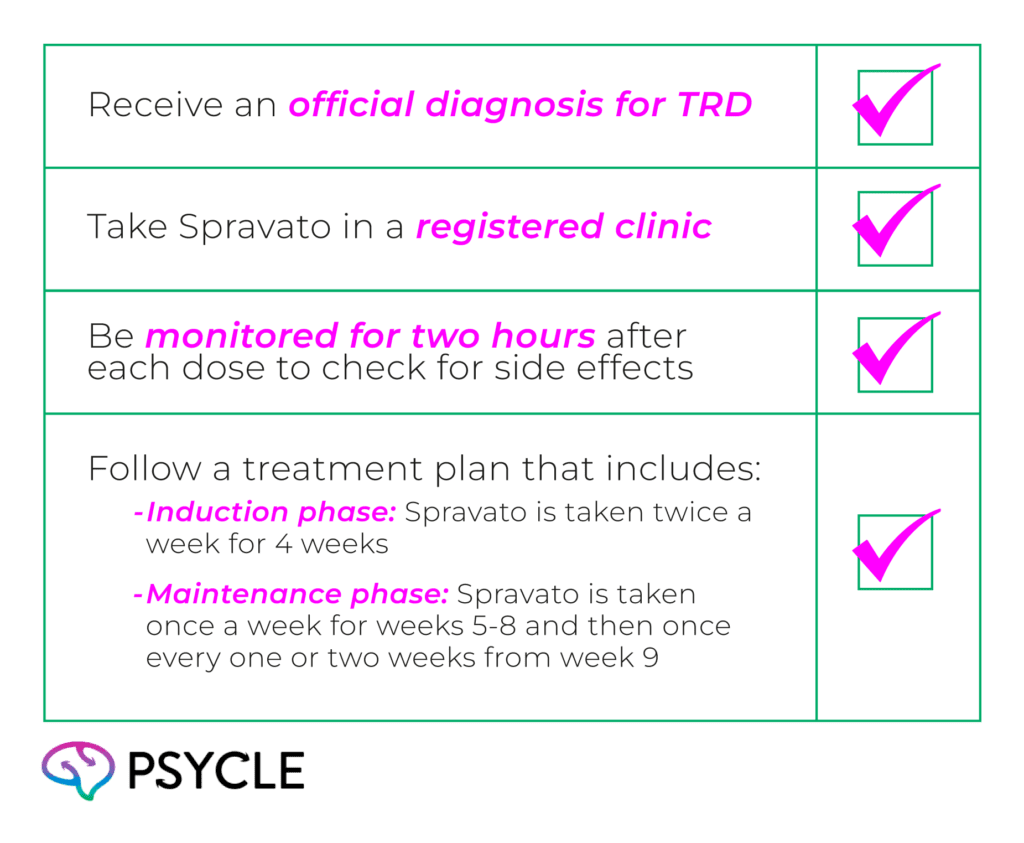
The safety plan for using Spravato in Australia is based on the European Union’s Spravato Risk Management Plan, which establishes strict guidelines to ensure patient safety.
Clinical Evidence Supporting Spravato’s Efficacy
Spravato’s approval and PBS listing are based on the drug’s safety and efficacy in clinical trials. One of the largest studies has been the SUSTAIN-1 trial, which included over 700 patients with TRD. In the study, all patients received Spravato plus a regular antidepressant for four weeks.
After four weeks of treatment, about 65% of patients showed a major improvement, meaning their depression symptoms had reduced by more than half. These patients, known as responders, continued using Spravato for up to a year to assess its long-term effectiveness.
By the end of 12 weeks, 52% of the responders were in remission, meaning their symptoms had mostly disappeared.
Side Effects and Risks of Spravato
While Spravato is an exciting breakthrough, it’s not without side effects.
Some common side effects include:
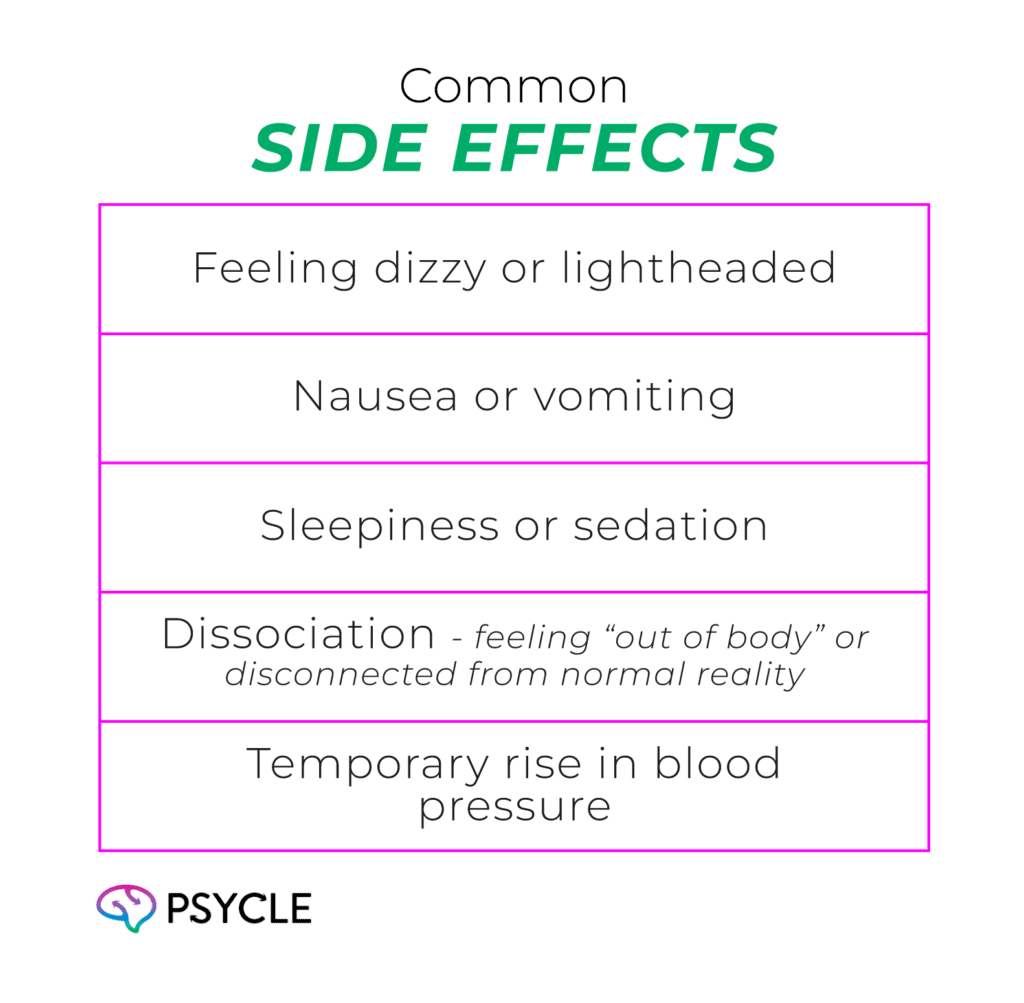
There are some more serious side effects and risks associated with Spravato, mainly when used in people with certain conditions. As such, patients may be prevented from receiving the medicine if they have the following:
- Uncontrolled high blood pressure
- History of hemorrhage
- Vascular disease
- Pregnancy or breastfeeding
The Future of Ketamine and Psychedelic Healthcare in Australia
Spravato’s addition to the PBS could be the start of a bigger shift in how Australia treats mental illness.
Australia is already ahead of the game when it comes to alternative treatments for mental health. In 2023, the country became the first in the world to allow psychiatrists to prescribe MDMA and psilocybin (the active ingredient in magic mushrooms) for certain mental health conditions.
While this type of psychedelic treatment is still very new and expensive, it shows that the mental health field is exploring new options. Moreover, with Spravato becoming more financially accessible, it’s possible that other psychedelic treatments could be covered by Australia’s benefits schemes in years to come.
More research and more funding will be needed, but Spravato’s PBS listing is a strong sign that Australia is taking alternative mental health treatments seriously.
FAQs
Is Ketamine Infusion Therapy Covered by Insurance in Australia?
Traditional ketamine infusions for depression are not covered by Medicare or the Pharmaceutical Benefits Scheme (PBS) in Australia. Patients typically pay the full costs for drug prescription and treatment services. While the cost of ketamine itself is relatively low–around $5 to $20 a dose–the price of consultation, administration, and monitoring adds a significant extra cost (around $350).
What are the Out-of-Pocket Costs for Spravato in Australia?
Even though Spravato is now listed on the Pharmaceutical Benefits Scheme (PBS), there are some out-of-pocket costs you’ll be expected to pay. Most clinics charge a monitoring or supervision fee for each Spravato session. These fees can range from $100 to $300 per visit, depending on the clinic.
Sources
- https://www.tga.gov.au/resources/auspmd/spravato
- https://www.theguardian.com/australia-news/2025/apr/28/nasal-spray-similar-to-ketamine-to-be-added-to-pbs-for-treatment-resistant-depression

